Articles from SpineX Inc.

VIVATRONIX Tech Pvt. Ltd. (Bangalore, India) and SpineX Inc. (Fremont, CA, USA) proudly announce that the Central Drugs Standard Control Organization (CDSCO), India’s national regulatory authority, has approved xStep (powered by SpineX) for clinical and home use in India.
By SpineX Inc. · Via Business Wire · October 9, 2025

SpineX Inc., a pioneering medical technology company developing innovative non-surgical spinal cord neuromodulation solutions, today announced the results of its global, multisite pivotal trial, CONTINENCE, evaluating the safety and efficacy of SCONE™ therapy for neurogenic bladder (NB) in individuals with paralysis due to spinal cord injury (SCI), stroke, or multiple sclerosis (MS).
By SpineX Inc. · Via Business Wire · April 15, 2025

SpineX Inc. has closed recruitment for its clinical trial of its proprietary SCONE™ device. The clinical trial—Spinal COrd NeuromodulaTor by SpIneX and ScoNE to Treat NeurogeniC BladdEr - SCONE “CONTINENCE” Clinical Study— began in May 2022.
By SpineX Inc. · Via Business Wire · April 8, 2024
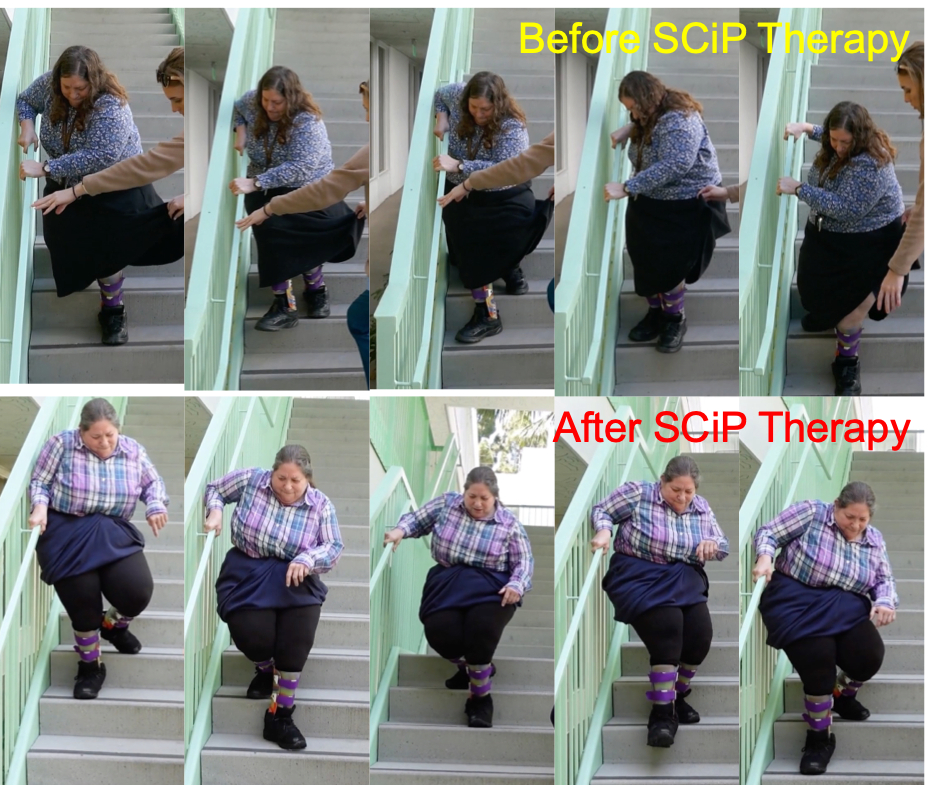
SpineX Inc. today announced groundbreaking results in its first in-human study treating an adult with Cerebral Palsy (CP). The study, published in the esteemed medical journal BioElectronic Medicine, demonstrates significant functional improvements in an adult with CP after treatment with the company’s proprietary non-surgical SCiP™ therapy.
By SpineX Inc. · Via Business Wire · January 3, 2024

SpineX Inc. is today announcing close to full recruitment -- over 80% -- of the patient cohort for its clinical trial of its proprietary SCONE™ device. SCONE is an innovative device that treats urinary incontinence allowing people living with neurogenic bladder due to spinal cord injury, multiple sclerosis, or stroke to live life on their own terms.
By SpineX Inc. · Via Business Wire · December 6, 2023

SpineX Inc., a clinical stage bioelectric medtech company, has had an important publication accepted in the prestigious Journal of Urology. The publication, entitled A Pilot Study of the Effect of Transcutaneous Spinal Cord Stimulation on Micturition-Related Brain Activity and Lower Urinary Tract Symptoms after Stroke shows improved bladder function and restoration of brain activity associated with normal voiding in stroke survivors who completed SpineX SCONE™ therapy.
By SpineX Inc. · Via Business Wire · November 9, 2023

SpineX Inc., a clinical stage bioelectric medtech company, is pleased to announce receipt of funding from the National Institutes of Health (NIH) through the Small Business Innovation Research Awards (SBIR). These funds will go towards completing a pivotal clinical trial to test the efficacy and safety of our proprietary SCiP™ device in children living with Cerebral Palsy (CP).
By SpineX Inc. · Via Business Wire · September 26, 2023
Cutting-edge neuromodulation research being conducted by the SpineX team is once again being recognized by the world’s leading scientists, with the publication this month of two more peer-reviewed publications, both in Frontiers.
By SpineX Inc. · Via Business Wire · July 27, 2023

SpineX Inc., a clinical stage bioelectric medtech company, today announced that the first patient has been enrolled in a pivotal trial evaluating the SCONE™ Device. The pivotal trial, known as SCONE trial, will evaluate the safety and effectiveness of the SCONE™ device for the treatment of Neurogenic bladder. This is the first large-scale pivotal trial testing a non-invasive spinal neuromodulation technology for the treatment of neurogenic bladder. The first patient was enrolled at Rancho Research Institute, the research arm of the prestigious and historic Rancho Los Amigos National Neurorehabilitation Center, Downey, California (https://www.ranchoresearch.org/).
By SpineX Inc. · Via Business Wire · June 28, 2022
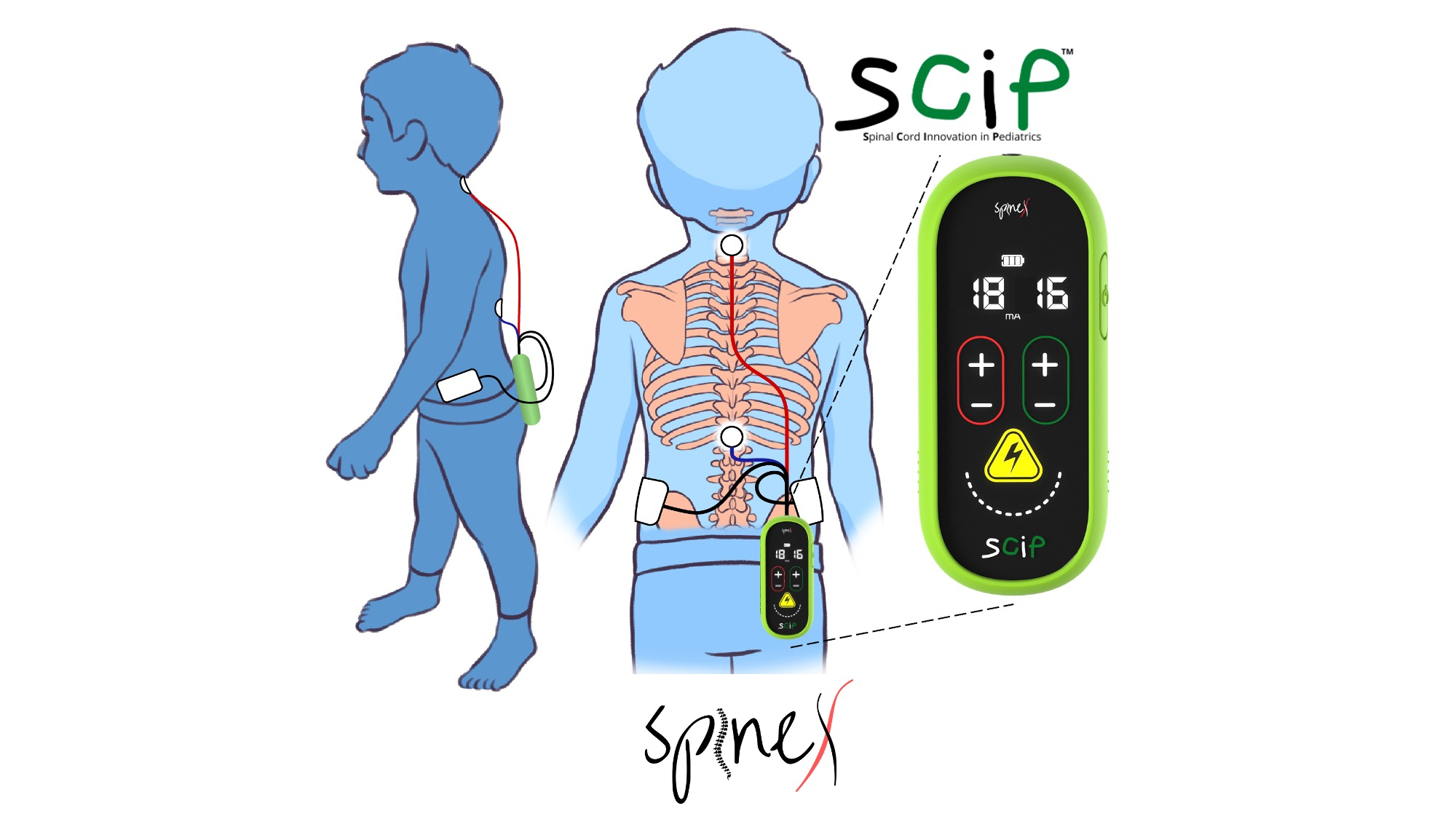
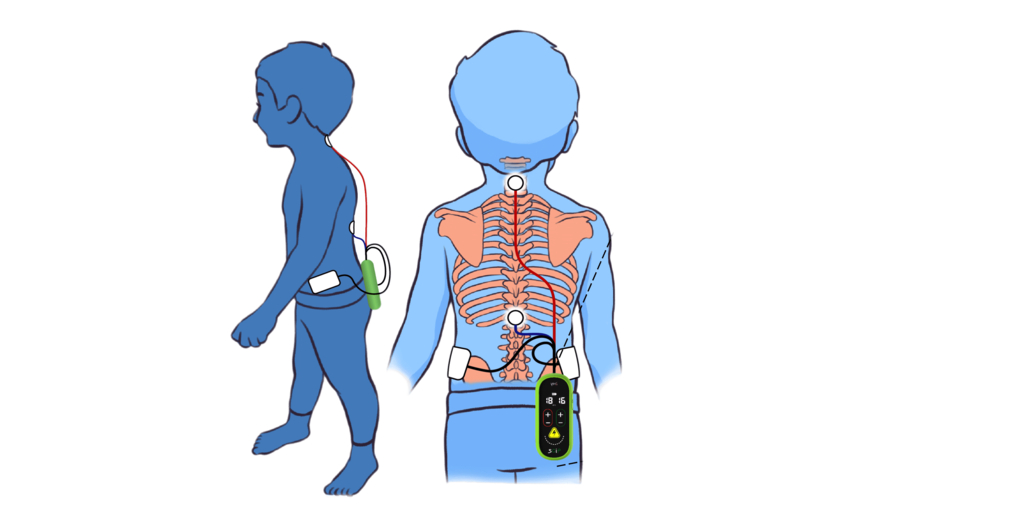
SpineX, Inc., a clinical stage medtech company today announced the groundbreaking results its first in human study in children with cerebral palsy. The study is published in the esteemed medical journal Nature Communications demonstrating unparalleled functional improvements with its proprietary non-surgical treatment SCiPTM (Spinal Cord Innovation in Pediatrics) in children with Cerebral Palsy (CP). “A pilot study combining noninvasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy”, led by Dr. Susan Hastings, PT, DPT and Dr. V Reggie Edgerton, PhD is the result of years of work championed by the two stalwarts of their respective fields. This study discusses how delivering non-invasive spinal neuromodulation, using SCiPTM, during Physical Therapy improved voluntary sensorimotor function in 16 out of 16 children over a wide range of ages and severities of CP. SpineX was awarded the Breakthrough Device Designation (BDD) from US FDA for SCiP and the proposed treatment of CP. In addition, SpineX has engaged with FDA to gain alignment on a proposed clinical trial to be conducted in 2023; the results of which are anticipated to lead to FDA clearance of the SCiPTM device for the treatment of CP.
By SpineX Inc. · Via Business Wire · October 5, 2022
